About Plasma
For over 25 years, the National Genetics Institute has provided the global plasma industry with source plasma donation screening services for human immunodeficiency virus (HIV), hepatitis A, B, and C viruses (HAV, HBV, and HCV), parvovirus B19, West Nile virus and other infectious agents to ensure the safety of the plasma supply chain. We have a long history of developing highly sensitive, multiplexed assays and in 2001, NGI received the very first FDA approvals for our HIV and HCV NAT assays.

NGI History
NGI provides the global plasma industry with comprehensive donor qualification and source plasma donation screening services including advanced genetic testing and viral marker testing (NAT and VMT). For over 25 years, NGI has provided the global plasma industry with source plasma donation screening services for human immunodeficiency virus (HIV), hepatitis A, B, and C viruses (HAV, HBV, and HCV), parvovirus B19, West Nile virus and other infectious agents to ensure the safety of the plasma supply chain. We have a long history of developing highly sensitive, multiplexed assays and in 2001, NGI received the very first FDA approvals for our HIV and HCV NAT assays. Our breakthrough technologies combined with unique FDA-approved pooling algorithms enable NGI to detect nucleic acid targets in individual samples and pools of hundreds of individual plasma samples (as large as 512), providing significant cost and turnaround time advantages to our global client base.
In early 2019, we are expanding our test menu to offer the most comprehensive plasma donor screening services in the industry including NAT, VMT and Ancillary testing. We take pride in our unparalleled customer service, exceptional turnaround times, technical expertise and innovative test menu.

Setting the Standards for the Plasma Screening Industry
Our Commitment to Testing Excellence
Taking pride in providing our customers with exceptional customer service, rapid turnaround times, unparalleled scientific expertise, and innovative laboratory solutions.
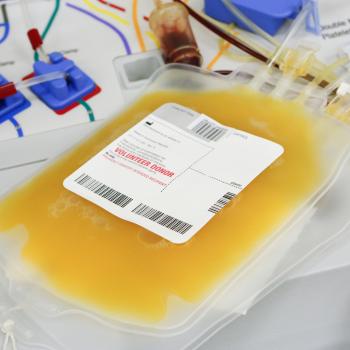
Established in 1991, National Genetics Institute has been an innovator in the areas of infectious disease nucleic acid testing and screening and has worked closely with regulatory authorities developing standards in blood and plasma screening. The first FDA approvals for UltraQual® HCV and HIV assays were received in 2001. Currently, NGI holds Biologics Licenses from the US Food and Drug Administration (FDA) for source plasma donor screening by qualitative detection of HCV, HIV, and HBV, and tests millions of plasma donations annually.
The NGI testing platforms are used by the global source plasma industry and specialty pharmaceutical product manufacturers to ensure that plasma-derived therapies are free from blood borne infectious agents. NGI’s advanced and comprehensive plasma screening services are compliant with relevant federal regulations and guidelines. As a part of the LabCorp® Specialty Testing Group, NGI has full access to the vast resources of our parent company and its subsidiaries to offer end-to-end laboratory testing solutions for the global plasma industry. As an example, our sister laboratory Viromed offers a full range of FDA-compliant donor confirmatory and supplemental testing.
Plasma Industry Timeline